This article is more than 1 year old
At the third beep, the Atomic Clock will be 60 ... imprecisely
The science that makes GPS (and a whole lot more) possible
Beat the clock
The amount by which time was predicted to dilate at velocities that might be achieved on earth was microscopic. To carry out an experiment to detect it, one would need clocks of fantastic accuracy that simply didn't exist, but the need for such clocks was suddenly clear.
At around the same time, quantum physics was emerging and one aspect of that was photo-electricity, which interested Einstein. The controversial nature of relativity was such that Einstein was awarded the Nobel Prize for his contribution to photo-electricity. Einstein argued that radiation interacting with matter would do so most strongly if the frequency of the radiation was proportional to the energy difference between the different states of excited matter. The constant of proportionality was Planck’s Constant.
Quantum physics held that atoms can only emit radiation in packets: absolutely fixed quanta of energy, or not at all. This may be just as well because, were it not for that, atoms could lose energy gradually and run down, which would be bad news for the universe. These packets of energy would in due course be called photons.
In 1917, Einstein essentially predicted stimulated emission of radiation, which would in due course lead to Masers and Lasers. Serendipitously, the tightly defined frequencies of radiation due to quantum effects would provide the precision needed to make accurate clocks. Testing Einstein’s relativity needed accurate clocks, and his photoelectric work hinted at the principles of how they might work, even if no one at that time knew how to make one.
The difficulty was such that an accurate atomic clock based on the frequency of quantum radiation would not be built until 1955, coincidentally the year of Einstein’s death. Getting stimulated atoms to radiate is a piece of cake. Simply sprinkling common salt onto a naked flame will result in a pleasing display of sodium yellow light, which has a wavelength of 5893 Ångström Units. All you have to do is to divide down the frequency of the radiation and you have a clock.
As early as 1907 it was recognised that the metre simply wasn't accurate enough for scientific purposes as it was just a random lump of metal kept in Paris. The Ångström Unit was defined as a specified number of wavelengths of the emission from cadmium, which of course is determined by quantum behaviour. In 1960 the metre would be re-defined as 1010 Ångström Units, the wavelength of Sodium yellow became 589.3 nanometres and Anders Ångström’s contributions to physics would no longer be commemorated.
The problem with visible light emitted by atoms is that the frequency was just too high for early electronic equipment to count. The Second World War had provided a massive stimulus for the development of radar and this led to devices such as cavity magnetrons that could operate at microwave frequencies. Suppose, then, that an element could be found that had a quantum energy gap low enough to emit in the microwave region? It might then be possible to obtain quantum radiation that had a low enough frequency for realisable electronic equipment to lock to it.
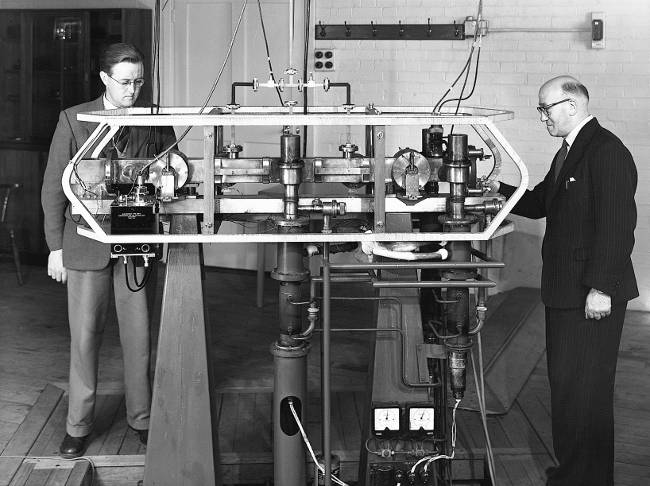
Essen and Parry with Caesium resonator. Pic: Courtesy of NPL
Two candidates were found, Rubidium and Caesium. Both are alkali metals, sharing the same column or group in the periodic table. The periodic table gets its name because as the number of electrons in elements increases, they fill up shells. Elements with the same number of electrons in the outer shell tend to have similar characteristics. Rubidium and Caesium both have one electron in their outer shell. All the other electrons are sitting fat, dumb and happy in nice stable filled shells, so if you poke the atom with a stick it will be the odd one in the outer shell that responds.
The lowest frequency of quantum radiation from Rubidium is about 6.8GHz and that from Caesium is about 9.2GHz. The first Caesium clock was built by Essen at the National Physical Laboratory in UK in 1955. It combined microwaves and Caesium atoms in such a way that the natural quantum frequency of the Caesium could be divided down and output as an electronic clock signal.
