Experimental brain-spine computer interface helped a paralyzed man walk
Pioneering research effectively reconnects patient's motor cortex with his spinal cord
Comment A paper in Nature reveals how a brain implant and computer-controlled prosthetic helped a paraplegic man in his recovery from a partially severed spinal cord.
Some two years after the research project began, the newly published study describes how some experimental technology from the Ecole Polytechnique Fédérale de Lausanne (EPFL) in Geneva, Switzerland, has not only managed to electronically reconnect the patient's brain with the lower part of his spinal cord, enabling him to stand, walk, and even climb stairs – but it's also helping with his rehabilitation.
The brain-spine interface (BSI) implant used in this case seems to be helping him to grow new nerve connections. It's taken over a year of hard work to get this far, and he is now able to walk short distances even when the prosthetic is turned off. And this is all more than a decade after he suffered the crippling injury to his back.
By prosthetic, we mean a backpack of electronics that receives signals from the man's brain – his cerebral cortex – figures out the movement he was trying to make, and then fires signals to his muscles via pulse generators attached to his lower back, to achieve that movement. The prosthetic effectively patches over the break in his spinal cord – delivering messages that otherwise wouldn't get through, allowing him to move by himself again.
The prosthetic includes the implant in his brain as well as the signal generators.
How it was done
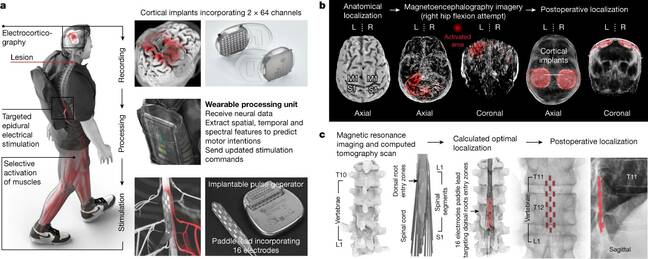
Although it uses some tech that's already out there, this is not off-the-shelf kit. The intrepid patient, Dutchman Gert-Jan Oskam, volunteered to have two five-centimeter (two-inch) holes cut out of his skull to place a couple of permanent brain implants to sit over paired regions of his motor cortex. Careful measurement and study showed the researchers these were the regions that used to control his hip and leg muscles.
The implants are WIMAGINE units, the inventors of which call them ElectroCorticoGrams. These sit on top of the dura mater – the protective membrane around the brain – so that they're not actually directly contacting the gray matter. That means that they should be safe for long-term implantation. Which they'd better be, because they are mounted on titanium plates as thick as the bone of the cranium.
Overview ... The design, technology and implantation of the BSI, as detailed in the Nature study. Credit: Lorach et al. Click to enlarge
Your humble vulture here on The Reg FOSS desk was last month the lucky recipient of more than 30 new non-brain implants into his own skeleton – in this instance, in his right forearm. These, like most pins and plates for broken bones, are made of surgical steel – a material with a very smooth surface. Titanium, like aluminum, has a porous surface layer of oxidized metal so that bone grows into it. Surgical steel implants can be fairly straightforwardly removed again if they're no longer needed, while titanium ones are usually there forever.
To transmit the signals from the cerebral cortex out of his skull, Oskam must wear a pair of transmitter-receivers over the implants. These are mounted on a headband and resemble a pair of headphones that sit on top of his head.
Each pair contains two antennas: one powers the electrodes inductively, via a high-frequency signal, and the other receives data from the electrodes over UHF. They pick up the nerve impulses from his motor cortex, and send them over a cable to a laptop in his backpack, which interprets the signals, works out what body part he is trying to move, and generates simulated nerve impulses to command his hip and leg muscles.
Another implant, derived from an Activa RC deep-brain stimulation unit and a Specify 5-6-5 electrode located inside Oskam's spinal column next to his spinal cord, transmits the synthetic nerve signals into the lumbar region of his spinal cord, where the spinal nerves branch off to his legs. From there, the artificial nerve signals travel down his spinal nerves, into the formerly paralyzed muscles, allowing him to stand up, take steps forward, moving his ankles to raise his feet as necessary to clear obstructions. After a lot of practice sessions and rehab, he's not just able to walk on level terrain using crutches, but even to climb stairs or a ramp.
Several aspects of the research are especially noteworthy. One is that this is not the first experimental treatment that Oskam has volunteered for. He also participated in an earlier experiment, in which electrodes in his legs directly stimulated his leg muscles. In combination with orthotic splints on his lower legs to hold his ankles and keep his feet straight, this earlier treatment did enable him to walk for short distances on a flat surface – but that was all.
Despite lots of therapy and practice, he was unable to to do more than this. Although the team had hoped that it might help to regain more functionality, it didn't happen.
Second, with the new system his control and balance are so good that the university team constructed a special separate standalone version of the BSI. Whereas the lab version is partly located in a backpack and lets him walk with crutches, this is a take-home version – it mounts onto a wheeled walking frame, allowing him to use it outside of the laboratory.
Third, and perhaps most encouragingly of all, since he's been using the BSI, he has regained some of the lost function and movement in his legs – which the earlier experiment sadly didn't help. The videos in the Nature paper show that in the system's early days, turning off the implant left him immobilized – literally unable to take another step.
However, he is now able to walk for very short distances without the aid of the prosthesis. That suggests that with regular use over a sustained period, new connections are reforming in his spinal cord and reestablishing connections with his brain.
- Funnily enough, FDA forbids Elon Musk's Neuralink human experiments
- Elon Musk's Neuralink probed over pathogen transport
- Man paralyzed from neck down uses AI brain implants to write out text messages
- Shocking. Lightning strike knocks out neuro patient's brain implant
This is borne out by earlier work, such as this 2020 study which found that artificial stimulation using the same sort of spinal electrode array helped connections to regenerate. The difference in the new study is the origin of the signals: from directly scanning the motor cortex in real time.
These are very early days. The BSI isn't sending the original, interrupted nerve impulses – the hardware is generating its own artificial nerve impulses, trying to simulate the ones that no longer pass down his damaged spinal cord. Also, this isn't a two-way link – it can't relay sensory impressions back to his brain.
This Register scribe has friends with spinal injuries, who told him that learning to walk afterwards compares with walking on stilts: when you can't feel your legs, it's substantially more difficult.
For now, this research is being conducted on a single, brave volunteer – and we salute him for undergoing major, and very invasive, surgery, for the sake of science and helping others. While it's already improved his quality of life, it's mainly helping the academic team to develop the technology.
Along with other research, which is for example driving down the cost of exoskeletons to help paralyzed people stand and walk, some of the science-fiction promises of technological solutions to damage to our fragile meat-sacks seem to be starting to come true. ®
